Introducing test automation in a GxP governed ecosystem. Find out how Sii Poland used Tricentis Tosca to build a healthcare client’s new AI system
Sii experts supported one of the world’s largest biotechnology companies by building a solution that replaces a business-critical pharmacovigilance system. By introducing test automation with Tricentis Tosca, Sii was able to reduce the company’s costs and time spent on testing the new solution.
Sii’s biotech client is running an innovation program that aims to build a solution based on artificial intelligence. The program will enable a highly automated, end-to-end process for the identification, characterization, and reporting for drug adverse events by applying cognitive computing and advanced analytics.
This complex undertaking includes three major applications delivered by external vendors, as well as custom developed platforms for the purpose of integration, reconciliation, and aggregate monitoring. The program uses a variety of technologies like Snowflake, PostgreSQL, API, ETL, MuleSoft, Spotfire, Tableau, AI, and LP. Applications within the program scope deal with patient personal data, and directly impact customer core business continuity as well as regulations established by the relevant health authorities.
How to automate in a GxP governed business
The client needed to introduce test automation at the early stages of the project to reduce costs and time spent on testing the solution. But the introduction of test automation is not so simple in a highly regulated GxP environment. The biotech company reached out to Sii for help because of its testing experience and expertise. Sii is the leading IT, engineering, and BPO professional service provider.
The first thing that needed to be addressed was the client’s extensive technology landscape and variety of systems that required integration. An automation solution should have:
- Both front and back-end testing capabilities
- The ability to increase automation speed and lower maintenance effort
- The ability to cover the management of sensitive test data
- An easy way to scale up testing and run testing in parallel across distributed infrastructures and virtual machines
Secondly, the solution should deliver robust end-to-end automated testing for validated products that comply to pharma regulations. This step requires developing a wide range of documents and procedures including defining user requirements (URS), associated risk assessments (SRA, FRA), architecture, installation configuration, and verification. All of the mentioned deliverables must be compliant with internal operating procedures as well as overall industry standards.
During the planning phase, Sii experts assessed several test automation solutions including an enterprise testing tool and a custom opensource tool based on Robot Framework and Python. Sii’s architects supported this assessment and delivered a detailed solutions analysis and several technical proof of values to the client.
In the end, Sii selected Tricentis Tosca to cover the test automation of the biotech client’s program. Tosca satisfies all of the testing requirements and supports the versatile underlying technologies on an enterprise level with outstanding ROI.
Why Tricentis Tosca?
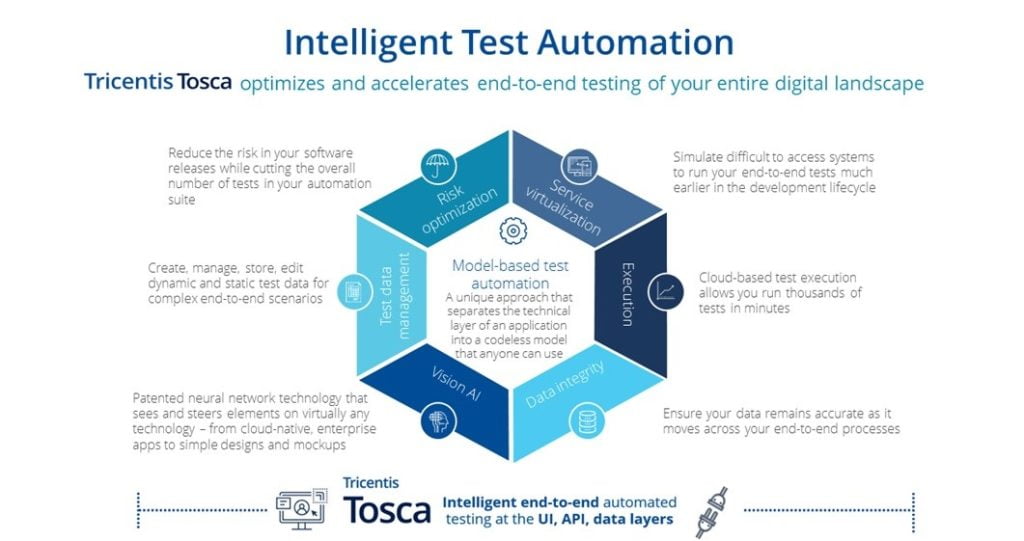
Tricentis is a global leader in enterprise continuous testing. Tricentis Tosca accelerates testing with a script-less, no-code approach for end-to-end test automation. Tosca is recognized as the industry leader by customers in the healthcare industry and by the top three industry analysts (Gartner, IDC, and Forrester).
With support for over 160+ technologies and enterprise applications, Tosca is able to cover the complex technologies used in the biotech client’s project. Tosca provides resilient test automation for any use case and many of the supporting capabilities required by the program:
Additionally, Tosca met all the client’s GxP regulations for a robust end-to-end automated testing solution for validated products that complies with pharma regulations. Tricentis solutions enable clients in FDA-regulated industries to comply with the electronic record and signature requirements of 21 CFR Part 11.
Tosca GxP compliance qualification
Sii’s Poland location served as the Tosca implementation partner and supported the GxP compliance qualification process. To ensure that Tricentis Tosca was compliant with healthcare and pharma regulations, the Sii team of experts conducted these activities to validate the tool:
- System Risk Assessment – to calculate the overall risk level for the new solution
- User Requirement Specification – to set the scope of functionalities to be used by testers during User Acceptance Testing
- Design Specification – to document more technical features of Tosca
- Assurance Plan – to describe Test Plan and other activities needed for the qualification process
- System Operator Handbook and Technical Operating Procedure – created as an instruction for using the tool and the entire solution infrastructure
- Instructions for setup and configuration of Tosca server – included Common Multi-user Repository, License Server, and Tosca Agents
- Installation Verification scenarios – created to test all the components of the infrastructure
- Test scenarios – to test URS requirements
- User Acceptance Testing – conducted to validate the entire solution
- WebForms – to handle requests and incidents related to the Tosca solution
- gSite – created to be a ‘one-stop shop’ for all information related to Tosca for the client
After several months of implementation, detailed analysis, and document and process creations, Tricentis Tosca was successfully validated and allowed as a test automation solution to be used in the client’s projects.
Introducing innovation in pharma testing and validation
Validating Tosca and bringing a model-based test automation approach to the biotech client triggered new opportunities to innovate and sped up the testing process. Such pharma activities are what Sii Healthcare & Life Science is known for in the industry and its Competency Center experts play a crucial role in advising clients.
— Competences are important, however domain experience in connection with tools, bring project implementation efficiency expected by demanding customers — says Wojciech Drescher, Head of Healthcare & Life Science. — The healthcare sector is growing rapidly and tools like Tricentis Tosca can optimize end-to-end testing of the entire pharmaceutical digital landscape. Its codeless, AI-powered approach accelerates innovation across an enterprise by taking the bottlenecks out of testing and the risks out of software releases — adds Wojciech.
— Every healthcare organization strives to optimize patient care and outcomes, but some achieve greater success than others. From an IT perspective, this requires impeccable data and flawless end-to-end digital experiences for patients, partners, and employees. That’s when Tricentis comes together with partners like Sii Poland to implement automated end-to-end testing — says Peter Szedlacek, Vice President EMEA Alliances and Channels Tricentis.
Get to know Sii Poland’s offer for the healthcare sector and testing services. If you are interested in automating testing processes in your company, contact us.